PIPELINE
What is Dry Eye Syndrome?
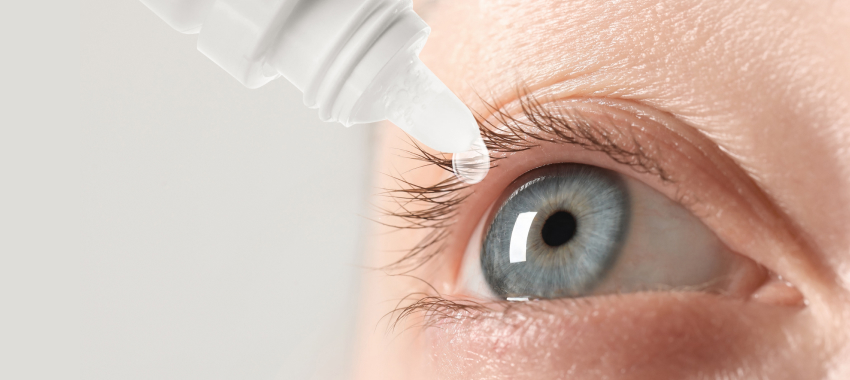
Dry eye involves a decrease in the amount and quality of the tear layer that moisturizes the eyes. The tear layer functions to maintain a soft, comfortable, and protective eye surface, but damage to the eye surface, resulting in abnormal homeostasis of the tear layer may undermine this function. Symptoms include dry eyes, burning, and blurred vision.
In addition, due to various environmental factors, such as riding in a vehicle, TV, blowing dry air from a heater or air conditioner, dryness in the winter, mobile phone/computer use, and dust, the components that make up these tears are imbalanced by either lack of tears or evaporation. This breaks up the tear film and damages the eye surface, causing dry eye syndrome.
Development Stage
| Pipeline | Treatment Areas | Indication |
Initial
Development Clinical Trial Phase 1 Clinical Trial Phase 2 Clinical Trial Phase 3 |
|
|---|---|---|---|---|
|
RGN-259
|
Ophthalmic desease
|
Dry Eye Disease
|
|
|
- RGN-259 : An ophthalmic solution containing Thymosin Beta 4 (timbetasin) for ocular diseases
- Preparing for the Phase 3 ARISE-4 clinical trial of the dry eye disease treatment (RGN-259) through the U.S.-based joint venture, ReGenTree, LLC.


DED (Dry Eye Disease)
ARISE (A Phase III Study of RGN-259 Identifying Safety and Efficacy)
